Home>Updates
Precision laser system by JNJ Vision enters Chinese market
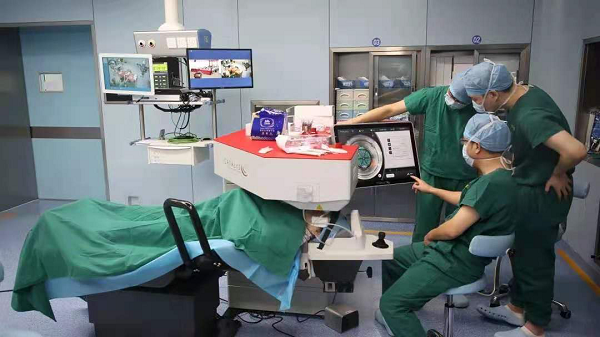
Medical staff use the Johnson & Johnson Vision's Catalys system during surgery.
Johnson & Johnson Vision's Catalys system, featuring a femtosecond laser designed specifically for cataract surgery, gained approval from the National Medical Products Administration (NMPA) on Jan 26, becoming the second medical device product using real-world data to enter the Chinese market.
The approval marks a new breakthrough in the clinical real-world experimental research of Hainan Boao Lecheng International Medical Tourism Pilot Zone.
The integrated laser scanning system is mainly used in cataract surgeries to remove the lens of the eye, including conducting an anterior capsulotomy and making single-plane and multi-plane arc incisions into the cornea.
Featuring high accuracy, high precision and high levels of safety, the system is able to create incisions in the cornea automatically after being programmed, and the accuracy can reach the size of a quarter of a hair. It can reduce the damage to the cornea, iris and capsular bag to a large extent during surgery.
Thanks to Lecheng's policy advantages in licensed operations, the system has already achieved remarkable outcomes in treating Chinese cataract sufferers. The first surgery applying the system was conducted in November 2019, and more than 100 patients have benefitted from the new technology.
Chen Wei, director of the International Eye Center of Boao Super Hospital and person in charge of the real-world research of the Catalys system, said that Lecheng will be a channel for globally innovative ophthalmology technologies to enter the Chinese market. The appearance of the Catalys system on the Chinese market will bring benefits to cataract sufferers, allowing them to enjoy advanced medical devices without traveling abroad.
Chen added that the center will strive to become the window for introducing globally innovative ophthalmology technologies to China and an innovative platform for the research, development, transformation and application of advanced ophthalmology technologies.
Gu Gang, director of the Hainan Boao Lecheng International Medical Tourism Pilot Zone Administration, said that the Catalys system's success in entering the Chinese market is a new outcome that showcases the support from the NMPA towards the construction of the Hainan Free Trade Port, as well as the integrated innovation jointly made by different government departments.
Gu noted that more global leading medical institutes and manufacturers in medical devices and medicines will be attracted to Lecheng, and that Lecheng will devote efforts in areas such as hosting a real-world data and evidence research conference to make more contributions to turning China into a global authority on the regulations and technologies of real-world research.

 Video: Foreign envoys send best wishes to Hainan FTP
Video: Foreign envoys send best wishes to Hainan FTP Hainan Xinshengquan International Cell Therapy Hospital
Hainan Xinshengquan International Cell Therapy Hospital  Neology Stem Cell Anti-Aging Hospital
Neology Stem Cell Anti-Aging Hospital  Boao Yiling Life Care Center
Boao Yiling Life Care Center 